The health of the environment is important, and a significant part of making our world safer involves making our world green. For many people, recycling is an easy way to help the environment, and that begins with placing plastic, paper, and glass into their respective curbside bins.
Glass, in particular, can be endlessly recycled with no loss of purity, and recycling it saves raw materials, reduces the demand for energy, and helps cut CO2 emissions. Just consider these recycling statistics:
- One ton of carbon dioxide is reduced for every six tons of recycled container glass used in the manufacturing process.
- Recycled glass is substituted for up to 95% of raw materials.
- Energy costs drop about 2–3% for every 10% cullet used in the manufacturing process.
However, not all glass is the same, so separating it correctly in the recycling process can be a challenge. Here, we’ll explore the glass recycling and sorting process and provide techniques to improve it.
Common Contaminants in Glass
Glass used in bottles and containers is called soda glass. This type of glass is recycled in a closed-loop system (meaning that it’s recycled back into soda glass), which saves raw materials needed to create new glass.
The environmental benefits of glass recycling are particularly advantageous because, unlike plastic, glass can be reused and recycled indefinitely, provided it contains no contaminants such as non-glass materials and unrecyclable glass ceramic material.
Unrecyclable glass materials such as ceramics, crystal, and clay are increasingly common in a variety of products, including garden pots, cookware, manufactured goods, and smartphones. These materials can even contain contaminants—including lead—that are harmful to our health.
Even if no harmful contaminants are present in the unrecyclable glass ceramic, most have different chemistries and a higher melting point than regular soda glass. This can potentially cause damage to the glass furnace and cutting systems, as well as create impurities in new glass material.
Glass bottles with impurities also have a greater risk of shattering when being used or transported. This can lead to potential product safety and manufacturing issues further down the supply chain.
As more new glass containers are made with recycled glass, it’s especially important to separate contaminated waste from the recyclable glass in a fast, affordable, and reliable manner.
But, how? To answer this question, let’s explore the modern recycling process to understand how to make glass sorting more practical and efficient.
Glass recycling process
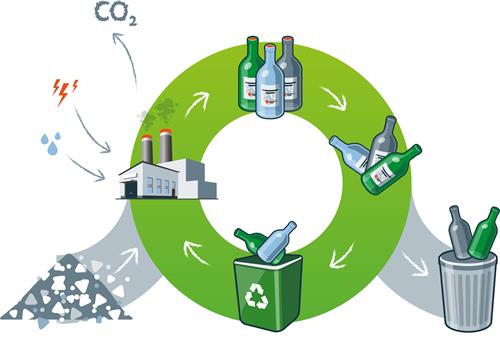 Sorting Through the Modern Recycling Process
Sorting Through the Modern Recycling Process
In modern single-stream recycling, all the recyclable material gets dumped on a conveyor, where it undergoes a multistep separation process. Facilities use multiple screening and separation techniques to sort out various contaminants based on their physical characteristics.
For example, magnetic sorting is used to filter out ferrous alloys. Plastics, papers, and non-ferrous alloys can be sorted out via a combination of density sorting techniques (e.g. vibrating screens) and optical screening.
At the end of the process, the remaining glass material is crushed into small pieces called cullet. However, certain types of unrecyclable materials, such as glass ceramics, crystal, and clay garden pots, may still be present in the cullet and will need to be sorted out. The cullet then moves on to the reclaiming facility where this unrecyclable glass is removed.
Historically, glass recyclers used magnetic or visual/infrared sorting on certain types of glass materials. But, there are some drawbacks to these methods. For example, certain ceramic glasses (like transparent cookware) and recyclable glass have very similar physical and optical properties. As a result, they are distinguished only by their chemical composition.
So, how do we efficiently separate safe, recyclable glass from unsafe materials? The answer is X-ray fluorescence (XRF).

Glass cullet stream
What is X-Ray Fluorescence?
Simply put, XRF is a spectroscopy technique that can be used to determine the material chemistry of a sample. It utilizes the physical phenomenon of photoelectric absorption and emission, whereby bombarding a material with energetic X-rays leads to the generation of secondary fluorescent X-rays.
These secondary X-rays will have energy that is characteristic of the elements that produce them. Using a specialized detector, it’s possible to capture and measure these fluorescent X-rays and measure their energy, thus generating a spectra that can be mathematically analyzed to determine composition.
One of the key advantages of utilizing XRF technology over other spectroscopy techniques is that it’s completely nondestructive. It can also be used to test material “as is” — with little to no sample preparation.
What is Handheld XRF?
Historically, XRF testing has been done on large benchtop and laboratory systems and required careful sample preparation. These days, the same kind of analysis can be accomplished rapidly using handheld XRF technology, such as Olympus Vanta™ analyzers.
Handheld XRF uses the exact same technique as the large benchtop systems, which is possible because it uses a low energy and compact X-ray tube source. These portable XRF analyzers are already used regularly in a variety of industries, including scrap metal recycling, mineral/metal mining, and material QA/QC.
In recent years, handheld XRF devices have also been deployed in other kinds of recycling and material recovery operations, such as the recovery of precious metals from automotive catalysts and consumer electronics.
The Advantages of Using XRF for Glass Material Processing
Because of its ability to directly measure composition, XRF offers a distinct advantage over other techniques to sort out non-recyclable glasses and ceramics from normal soda glass.
For example, a handheld XRF analyzer can recognize ceramic identifiers, such as zinc, titanium, and lead in fragments of material as small as 1 mm (0.04 in.) diameter in as little as one to three seconds with a single trigger pull. Once identified, these materials can then be manually removed from the safe, recyclable glass.
Beyond its analytical capabilities, handheld XRF technology is designed to work in industrial environments. It also offers several other features that can help ensure productivity and speed in a material processing facility.
Wireless and cloud connected instruments can ensure that data collected from the XRF analyzer is uploaded automatically to process control systems after each test. It also enables facility managers to monitor instrument usage and performance remotely, as well as customize instrument configuration for each operator or task.
Sorting Glass Cullet with High-Speed, In-Line XRF Systems
While handheld XRF analyzers will more than suffice for small batches of cullet, a large glass recycling plant can process more than 20 tons of cullet per hour. This makes manual separation of the identified unrecyclable glass impractical. Fortunately, the XRF technique can also be scaled to high-speed, in-line systems.
For instance, Olympus’ X-STREAM™ in-line X-ray fluorescence analyzer uses automated, high-volume technology to rapidly and accurately sort glass cullet. In fact, this system can process as much as 28 tons of glass cullet per hour. It also uses multiple detector arrays so that even small fragments can be reliably separated.
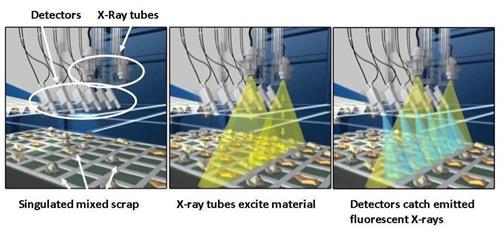
The X-STREAM in-line system sorts glass cullet as it passes under the arrays and separates contaminated fragments from safe glass using a blast of air. With the contaminated glass separated from the recyclable glass, the endless cycle of glass recycling can safely continue.
Dillon McDowell is an applications scientist, analytical instruments for Olympus, an ISRI member.